
#CATHODE RAY EXPERIMENT FARADAY TV#
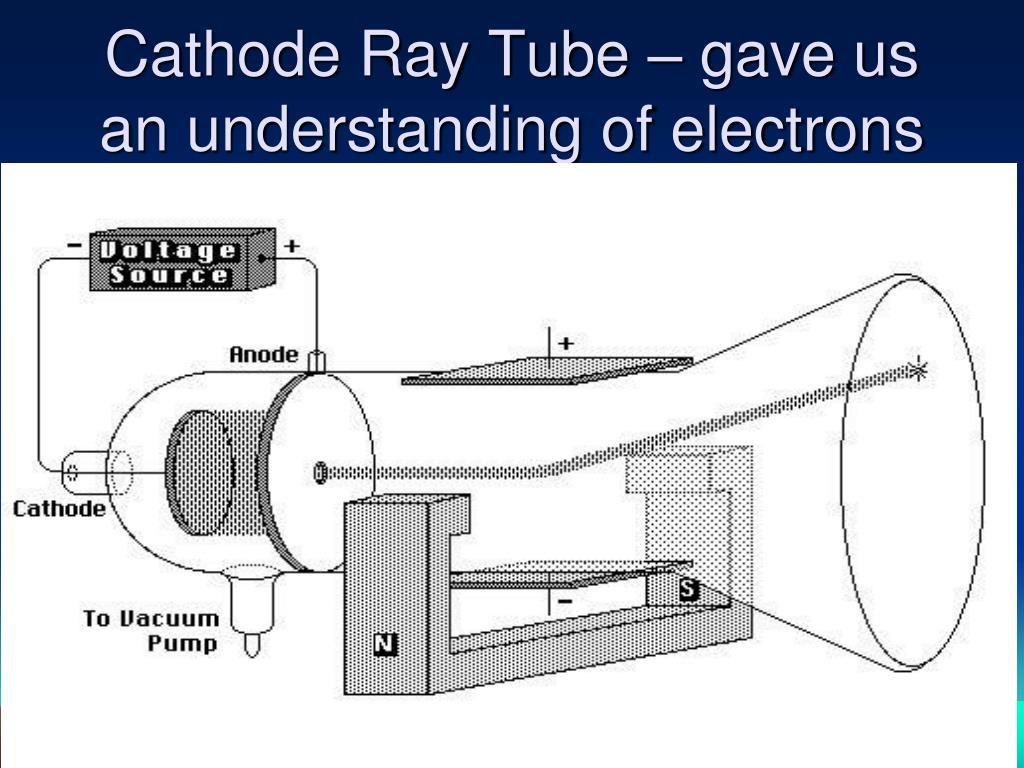
When we talk of Cathode Ray Tube (CRT) you would be reminded of our old box type TV or the big bulky desk top monitor. Let us start our journey with introduction to cathode ray tube (CRT) which is an electric discharge tube which scientists used for many discoveries related o electricity and atomic structure. I hope you will find this journey exciting !!! 1.1: Cathode Ray Tube (CRT) These are the same tubes which help us to watch films & spots on old box type TVs. These tubes were to lead into some exciting discoveries like X-rays, electrons and of course radiation. The journey will start around 1846 when Faraday had started working with ‘Discharge Tubes’. We will now take a journey from ‘Radiation 2 Neutrino’. RadiationĪnother major discovery of 19th century was ‘Radiation’ which led us to one of the most interesting particles ‘Neutrino’. These were one of the most influential discoveries starting from 1820 till date. We then went on to discuss how it helped to establish the theory of Big bang and creation of matter from pure energy.
We also discussed in some detail the electro-magnetic interaction including electro-magnetic spectrum, Black-body radiation, photo-electric effect which established the foundations of ‘Photon’. Photon is the carrier of electro-magnetic radiation including light. One of the major items that we covered in the previous discussions was “Photon”.
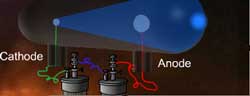
There are many other topics related to Big Bang and Dark Energy which we may come back to discuss as we go along. – MillikanĪfter discussing various aspects of Big bang, we will now move on to another topic ‘Neutrino’ which was repeatedly referred to in the discussions. Samsung galaxy y cas… on Best experiments – Inter…īest experiments… on Best Experiments – Inter… Best Experiments – Electron Diffraction – Black Body Radiation.Best experiments – Electron Diffraction – Photo electric effect.Best Experiment – Electron Diffraction – Bohr Atom.Best experiments- Electron diffraction – Broglie’s theory.Best experiments – Electron Diffraction – Davisson Germer Expr. 1927.To illustrate the effects of an electric field on a charged particle.To commemorate the discovery of the electron by J.As a result of his work, Thomson proposed a completely new model of the atom (Thomson’s Plum Pudding Model) that one of his students, Ernest Rutherford, would improve upon 10 years later.Ĭlick and drag the cursor to change the intensity of the voltage applied between the plates. This apparatus constitutes the first particle accelerator. Thomson, in 1897, isolated a new elementary particle carrying a negative charge – the electron. The very intense electric field that results from this accelerates the few ions present in the tube which, via collisions, ionize other particles. The lower the pressure, the more the electrons thus liberated and accelerated travel great distances until they strike the screen at the opposite end of the tube.īy studying the deviation of this beam, J. A high voltage (between 10 and 100 kV) is applied between two electrodes. Thomson’s second experiment involving the deviation of an electron beam in a vacuum tube, called a Crookes Tube.Ī partial vacuum (less than 10 -6 atm) is maintained in the tube.


 0 kommentar(er)
0 kommentar(er)
